Chem 101 Study guides, Class notes & Summaries
Looking for the best study guides, study notes and summaries about Chem 101? On this page you'll find 730 study documents about Chem 101.
Page 4 out of 730 results
Sort by
STRAIGHTERLINE CHEM 101 GENERAL CHEMISTRY GRADED EXAM 1 - APRIL 2022

-
CHEM 101 exam 4 FALL 2023_Straighterline
- Exam (elaborations) • 8 pages • 2023
-
- $17.49
- + learn more
Platinum has a face-centered cubic crystal structure and a density of 21.5 g/cm3 . What is the radius of a platinumSelect one: a. 69 pm b. 98 pm c. 139 pm d. 196 pm e. 277 pm Question 2 Correct 4.60 points out of 4.60 Flag question Question text Helium atoms do not combine to form He2 molecules, yet He atoms do attract one another weakly through Select one: a. dipole-dipole forces. b. ion-dipole forces. c. dispersion forces. d. dipole-induced dipole forces. e. hydrogen bonding....

-
CHEM 101 exam 1A FALL 2023 _Straighterline
- Exam (elaborations) • 8 pages • 2023
-
- $18.49
- + learn more
Which of the following activities is not a part of good science? Select one: a. Proposing a theory b. Developing a hypothesis c. Making quantitative observations d. Designing experiments e. Indulging in speculation Question 2 Correct 4.60 points out of 4.60 Flag question Question text What is a unifying principle that explains a body of experimental observations? Select one: a. Law b. Hypothesis c. Theory d. Phenomena e. Prediction Question 3 Correct 4.60 points out of 4.60 ...

-
Straighterline chem 101 exam 2 with questions and answers
- Exam (elaborations) • 11 pages • 2023
-
Available in package deal
-
- $15.49
- + learn more
Straighterline chem 101 exam 2 with questions and answers.The distinguishing characteristic of all nonelectrolyte solutions is that they Select one: a. contain ions. b. do not conduct electricity. c. react with other solutions. d. always contain acids. e. conducts heat. Feedback The correct answer is: do not conduct electricity. Question 2 Correct 4.60 points out of 4.60 Flag question Question text If aqueous solutions of Mg(C2H3O2)2 and LiOH are mixed, which insoluble precipitate ...

-
CHEM 101 exam 5 FALL 2023 _Straighterline
- Exam (elaborations) • 8 pages • 2023
-
- $17.49
- + learn more
Consider the following four solutions: NaCl in water Acetic acid in water Acetic acid in benzene Naphthalene in benzene Which of these solutions has the strongest solute-solvent interactions and the interaction is of which type? Select one: a. Acetic acid in water; hydrogen bonding b. Acetic acid in benzene; dipole-induced dipole interaction c. NaCl in water; ion-dipole interaction d. Naphthalene in benzene; London Dispersion Forces e. NaCl in water; hydrogen bonding ...
QUESTIONS AND ANSWERS

-
Module Two Chem 101 Problems Exam Questions And Answers 100% Solved 2024
- Exam (elaborations) • 25 pages • 2024
-
- $13.49
- + learn more
Module Two Chem 101 Problems Exam Questions And Answers 100% Solved 2024 Carbon dioxide is a _____ compound composed two types of _____ atoms. A. Molecular, metal B. ionic, metalloid C. molecular, nonmetal D. ionic, metal - answerC. molecular, nonmetal Carbon dioxide is a molecular compound composed of two oxygen atoms with covalent double bonds to a central carbon atom. Both C and O are to the right of the "staircase" on the periodic table, as nonmetals. Classify the following compo...

-
Chem 101 Final Exam Questions And Answers 100% Solved
- Exam (elaborations) • 13 pages • 2024
- Available in package deal
-
- $12.49
- + learn more
Chem 101 Final Exam Questions And Answers 100% Solved Determine the formula weight of KBr - answer119.00 amu Determine the mass in grams of 3.00 x 10^21 atoms of arsenic. (The mass of one mole of arsenic is 74.92 g.) - answer0.373 g Fill in the blank: A mole is defined as the number of carbon atoms in _____ g of pure ^12C. - answer12 How many atoms are in 1.2 mol of carbon? - answer7.2264 x 10^23 Which one of the following contains the most moles of oxygen atoms? A) 1 mol of Fe(C2H3O2)3...
CHEM 101-STRAIGHTERLINE EXAM 1 QUESTIONS & ANSWERS (2023/2024)(VERIFIED ANSWERS)
-
STRAIGHTERLINE CHEM 101 GENERAL CHEMISTRY GRADED EXAM 1 - APRIL 2022
- Exam (elaborations) • 14 pages • 2022
-
Available in package deal
-
- $10.49
- 1x sold
- + learn more
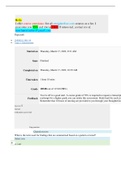
Page path • CHEM101_MH_V4 • Topic 3: Stoichiometry Started on Thursday, March 17, 2022, 9:21 AM State Finished Completed on Thursday, March 17, 2022, 10:39 AM Time taken 1 hour 18 mins Grade 105.80 out of 115.00 (92%) Feedback You’re off to a good start. A course grade of 70% is required to request a transcript. You’ve met that for the first exam already, but if you want to reattempt for a higher grade you can retake this assessment. Study hard for each exam, and consider using t...

That summary you just bought made someone very happy. Also get paid weekly? Sell your study resources on Stuvia! Discover all about earning on Stuvia