Maxim777
On this page, you find all documents, package deals, and flashcards offered by seller Maxim777.
- 86
- 0
- 0
Community
- Followers
- Following
2 Reviews received
86 items

When sensors in a car detect a collision, they cause the reaction of sodium azide, NaN3, which generates nitrogen gas to fill the air bags within 0.03
When sensors in a car detect a collision, they cause the reaction of sodium azide, NaN3, which generates nitrogen gas to fill the air bags within 0.03 s. 2NaN3(s) → 2Na(s) 3N2(g) How many liters of N2 are produced at STP if the air bag contains 138 g of NaN3? Express your answer with the appropriate units.
- Answers
- • 2 pages •
-
• chem 101
When sensors in a car detect a collision, they cause the reaction of sodium azide, NaN3, which generates nitrogen gas to fill the air bags within 0.03 s. 2NaN3(s) → 2Na(s) 3N2(g) How many liters of N2 are produced at STP if the air bag contains 138 g of NaN3? Express your answer with the appropriate units.

Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with HCl according to the following balanced equation. Al2O3(s) 6HCl(
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with HCl according to the following balanced equation. Al2O3(s) 6HCl(aq) → 2AlCl3(aq) 3H2O(l)
- Answers
- • 2 pages •
-
• chem 101
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with HCl according to the following balanced equation. Al2O3(s) 6HCl(aq) → 2AlCl3(aq) 3H2O(l)

a) When heated to 350 Co at 0.950 atm, ammonium nitrate decomposes to produce nitrogen, water, and oxygen gases: 2NH4NO3(s) → 2N2(g)
a) When heated to 350 Co at 0.950 atm, ammonium nitrate decomposes to produce nitrogen, water, and oxygen gases: 2NH4NO3(s) → 2N2(g) 4H2O(g) O2(g) How many liters of water vapor are produced when 24.9 g of NH4NO3 decomposes? Express your answer with the appropriate units. b) How many grams of NH4NO3 are needed to produce 19.5 L of oxygen? Express your answer with the appropriate units
- Answers
- • 3 pages •
-
• chem 101
a) When heated to 350 Co at 0.950 atm, ammonium nitrate decomposes to produce nitrogen, water, and oxygen gases: 2NH4NO3(s) → 2N2(g) 4H2O(g) O2(g) How many liters of water vapor are produced when 24.9 g of NH4NO3 decomposes? Express your answer with the appropriate units. b) How many grams of NH4NO3 are needed to produce 19.5 L of oxygen? Express your answer with the appropriate units

When 50.0 g of acetylene is allowed to react with excess oxygen, 75.0 g of CO2 are formed. What is the percent yield of carbon dioxide? 2C2H2(g)
When 50.0 g of acetylene is allowed to react with excess oxygen, 75.0 g of CO2 are formed. What is the percent yield of carbon dioxide? 2C2H2(g) 5O2(g) → 4CO2(g) 2H2O(g)
- Answers
- • 2 pages •
-
• chem 101
When 50.0 g of acetylene is allowed to react with excess oxygen, 75.0 g of CO2 are formed. What is the percent yield of carbon dioxide? 2C2H2(g) 5O2(g) → 4CO2(g) 2H2O(g)

The first step in the production of nitric acid is the formation of nitric oxide, NO, according to the following balanced equation. 4NH3(g) 5
The first step in the production of nitric acid is the formation of nitric oxide, NO, according to the following balanced equation. 4NH3(g) 5O2(g) → 4NO(g) 6H2O(g), ΔH = -906 kJ How many kJ are given off by the conversion of 34.0 g of ammonia?
- Answers
- • 2 pages •
-
• chem 101
The first step in the production of nitric acid is the formation of nitric oxide, NO, according to the following balanced equation. 4NH3(g) 5O2(g) → 4NO(g) 6H2O(g), ΔH = -906 kJ How many kJ are given off by the conversion of 34.0 g of ammonia?

F. Biology Ch. 2
F. Biology Ch. 2
- Answers
- • 10 pages •

HOMEWORK for Chapter 11: Gases
HOMEWORK for Chapter 11: Gases
- Answers
- • 25 pages •
HOMEWORK for Chapter 11: Gases

a) Calculate the total masses of the reactants in equation 4NH3(g) 5O2(g) → 4NO(g) 6H2O(g) Express your answer using
a) Calculate the total masses of the reactants in equation 4NH3(g) 5O2(g) → 4NO(g) 6H2O(g) Express your answer using four significant figures. b) Calculate the total masses of the products in equation above. Express your answer using four significant figures.
- Answers
- • 3 pages •
a) Calculate the total masses of the reactants in equation 4NH3(g) 5O2(g) → 4NO(g) 6H2O(g) Express your answer using four significant figures. b) Calculate the total masses of the products in equation above. Express your answer using four significant figures.

Question 1: a) Calculate the total masses of the reactants in equation 2SO2(g) O2(g) → 2SO3(g) Express your answer using five
Question 1: a) Calculate the total masses of the reactants in equation 2SO2(g) O2(g) → 2SO3(g) Express your answer using five significant figures. b) Calculate the total masses of the products in equation above. Express your answer using four significant figures. Question 2: a) Calculate the total masses of the reactants in equation 4P(s) 5O2(g) → 2P2O5(s) Express your answer using four significant figures. b) Calculate the total masses of the ...
- Answers
- • 3 pages •
Question 1: a) Calculate the total masses of the reactants in equation 2SO2(g) O2(g) → 2SO3(g) Express your answer using five significant figures. b) Calculate the total masses of the products in equation above. Express your answer using four significant figures. Question 2: a) Calculate the total masses of the reactants in equation 4P(s) 5O2(g) → 2P2O5(s) Express your answer using four significant figures. b) Calculate the total masses of the ...
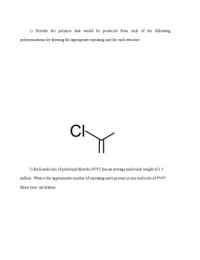
1) Provide the polymer that would be produced from each of the following polymerizations by drawing the appropriate repeating unit for each structure.
1) Provide the polymer that would be produced from each of the following polymerizations by drawing the appropriate repeating unit for each structure. 2) Each molecule of polyvinylchloride (PVC) has an average molecular weight of 1.5 million. What is the approximate number of repeating units present in one molecule of PVC? Show your calculation
- Answers
- • 4 pages •
1) Provide the polymer that would be produced from each of the following polymerizations by drawing the appropriate repeating unit for each structure. 2) Each molecule of polyvinylchloride (PVC) has an average molecular weight of 1.5 million. What is the approximate number of repeating units present in one molecule of PVC? Show your calculation

Introduction to MasteringPhysics
Lab to Determine the Outcome of Heredity