Maxim777
On this page, you find all documents, package deals, and flashcards offered by seller Maxim777.
- 86
- 0
- 0
Community
- Followers
- Following
2 Reviews received
86 items

Magnesium sulfate reacts with barium chloride according to the following balanced equation. If 1.20 g of magnesium sulfate is allowed to react with 10
Magnesium sulfate reacts with barium chloride according to the following balanced equation. If 1.20 g of magnesium sulfate is allowed to react with 10.0 g of barium chloride in a water solution, what is the theoretical yield of barium sulfate? MgSO4(aq) BaCl2(aq) → BaSO4(s) MgCl2(aq)
- Answers
- • 2 pages •
-
• chem 101
Magnesium sulfate reacts with barium chloride according to the following balanced equation. If 1.20 g of magnesium sulfate is allowed to react with 10.0 g of barium chloride in a water solution, what is the theoretical yield of barium sulfate? MgSO4(aq) BaCl2(aq) → BaSO4(s) MgCl2(aq)

The heat of reaction for the conversion of natural gas, methane, to carbon dioxide and water is -802 kJ/mol. How many kJ are given off by the combusti
The heat of reaction for the conversion of natural gas, methane, to carbon dioxide and water is -802 kJ/mol. How many kJ are given off by the combustion of 0.150 mol of methane? The reaction is: CH4(g) 2O2(g) → CO2(g) 2H2O(g)
- Answers
- • 3 pages •
-
• chem 101
The heat of reaction for the conversion of natural gas, methane, to carbon dioxide and water is -802 kJ/mol. How many kJ are given off by the combustion of 0.150 mol of methane? The reaction is: CH4(g) 2O2(g) → CO2(g) 2H2O(g)
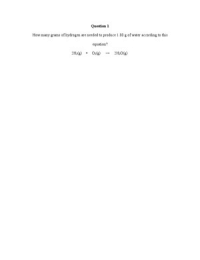
How many grams of hydrogen are needed to produce 1.80 g of water according to this equation? 2H2(g) O2(g) → 2H2O(g)
How many grams of hydrogen are needed to produce 1.80 g of water according to this equation? 2H2(g) O2(g) → 2H2O(g)
- Answers
- • 3 pages •
-
• chem 101
How many grams of hydrogen are needed to produce 1.80 g of water according to this equation? 2H2(g) O2(g) → 2H2O(g)

At 125 °C, the pressure of a sample of He gas is 345 mmHg. At what temperature (°C) will the pressure become 690. mmHg, if the volume remains constant
At 125 °C, the pressure of a sample of He gas is 345 mmHg. At what temperature (°C) will the pressure become 690. mmHg, if the volume remains constant?
- Answers
- • 3 pages •
-
• chem 101
At 125 °C, the pressure of a sample of He gas is 345 mmHg. At what temperature (°C) will the pressure become 690. mmHg, if the volume remains constant?

Below is a 1H NMR spectrum of isopentyl acetate. Assign all of the hydrogen signals of isopentyl acetate to the appropriate 1H NMR signals by writing
Below is a 1H NMR spectrum of isopentyl acetate. Assign all of the hydrogen signals of isopentyl acetate to the appropriate 1H NMR signals by writing the appropriate peak letter on the correct region of the template molecule shown. (10 points)
- Answers
- • 3 pages •
-
• chem 101
Below is a 1H NMR spectrum of isopentyl acetate. Assign all of the hydrogen signals of isopentyl acetate to the appropriate 1H NMR signals by writing the appropriate peak letter on the correct region of the template molecule shown. (10 points)

The most common source of copper (Cu) is the mineral chalcopyrite (CuFeS2). How many kilograms of chalcopyrite must be mined to obtain 415 g of pure C
The most common source of copper (Cu) is the mineral chalcopyrite (CuFeS2). How many kilograms of chalcopyrite must be mined to obtain 415 g of pure Cu? Express your answer to three significant figures and include the appropriate units.
- Answers
- • 3 pages •
-
• chem 101
The most common source of copper (Cu) is the mineral chalcopyrite (CuFeS2). How many kilograms of chalcopyrite must be mined to obtain 415 g of pure Cu? Express your answer to three significant figures and include the appropriate units.

Urea, NH2CONH2; ammonium sulfate, (NH4)2SO4; and ammonium hydrogen sulfate, NH4HSO4, are all common fertilizers. Rank the compounds in order from larg
Urea, NH2CONH2; ammonium sulfate, (NH4)2SO4; and ammonium hydrogen sulfate, NH4HSO4, are all common fertilizers. Rank the compounds in order from largest mass percent of nitrogen to smallest mass percent of nitrogen.
- Answers
- • 3 pages •
-
• chem 101
Urea, NH2CONH2; ammonium sulfate, (NH4)2SO4; and ammonium hydrogen sulfate, NH4HSO4, are all common fertilizers. Rank the compounds in order from largest mass percent of nitrogen to smallest mass percent of nitrogen.

For the following question, consider the following balanced equation. Mg3N2(s) 6H2O(l) → 3Mg(OH)2(s) 2NH3(g) Part A When
For the following question, consider the following balanced equation. Mg3N2(s) 6H2O(l) → 3Mg(OH)2(s) 2NH3(g) Part A When 2.00 mol of H2O react, how many grams of NH3 are produced? Part B How many grams of H2O are needed to produce 150. g of Mg(OH)2?
- Answers
- • 3 pages •
-
• chem 101
For the following question, consider the following balanced equation. Mg3N2(s) 6H2O(l) → 3Mg(OH)2(s) 2NH3(g) Part A When 2.00 mol of H2O react, how many grams of NH3 are produced? Part B How many grams of H2O are needed to produce 150. g of Mg(OH)2?

How many grams of chlorine gas are present in a 150. liter cylinder of chlorine held at a pressure of 1.00 atm and 0. °C?
How many grams of chlorine gas are present in a 150. liter cylinder of chlorine held at a pressure of 1.00 atm and 0. °C?
- Answers
- • 2 pages •
-
• chem 101
How many grams of chlorine gas are present in a 150. liter cylinder of chlorine held at a pressure of 1.00 atm and 0. °C?

Magnesium sulfate reacts with barium chloride according to the following balanced equation. How many moles of barium sulfate are formed from 10.0 g of
Magnesium sulfate reacts with barium chloride according to the following balanced equation. How many moles of barium sulfate are formed from 10.0 g of magnesium sulfate? MgSO4(aq) BaCl2(aq) → BaSO4(s) MgCl2(aq)
- Answers
- • 2 pages •
-
• chem 101
Magnesium sulfate reacts with barium chloride according to the following balanced equation. How many moles of barium sulfate are formed from 10.0 g of magnesium sulfate? MgSO4(aq) BaCl2(aq) → BaSO4(s) MgCl2(aq)

Introduction to MasteringPhysics
Lab to Determine the Outcome of Heredity